Imagine waking up every morning with deep, throbbing lumps under your arms, in your groin, or under your breasts. They don’t just hurt-they burn, leak, and come back again and again, no matter how hard you clean or what creams you try. This isn’t acne. It’s hidradenitis suppurativa (HS), a chronic skin disease that affects 1 to 4% of people worldwide, mostly women between 20 and 29. For years, doctors had little to offer beyond antibiotics, painkillers, and surgery. But now, a new wave of treatments is changing everything.
What Exactly Is Hidradenitis Suppurativa?
HS starts when hair follicles in areas with sweat and oil glands-like your armpits, groin, buttocks, and under the breasts-get blocked. The skin over them thickens, cells build up, and the follicle bursts. That triggers a fierce immune response. Your body doesn’t just fight infection; it attacks its own tissue, creating painful, deep nodules that turn into abscesses, then tunnels under the skin called sinus tracts. These don’t heal. They reopen. They scar. And they can last for decades.
It’s not caused by poor hygiene. It’s not contagious. And it’s not just a skin problem. HS is linked to higher risks of heart disease, depression, and metabolic issues. Many patients report feeling isolated, embarrassed, and exhausted. A 2023 survey by the HS Foundation found that 89% of people on biologic therapy saw their quality of life improve-but only after years of struggling alone.
Why Biologics Are a Game Changer
For decades, HS treatment was a cycle of trial and error. Antibiotics like clindamycin or doxycycline gave temporary relief. Hormonal pills helped some women. Surgery removed affected tissue, but scars came back. Nothing stopped the inflammation at its source.
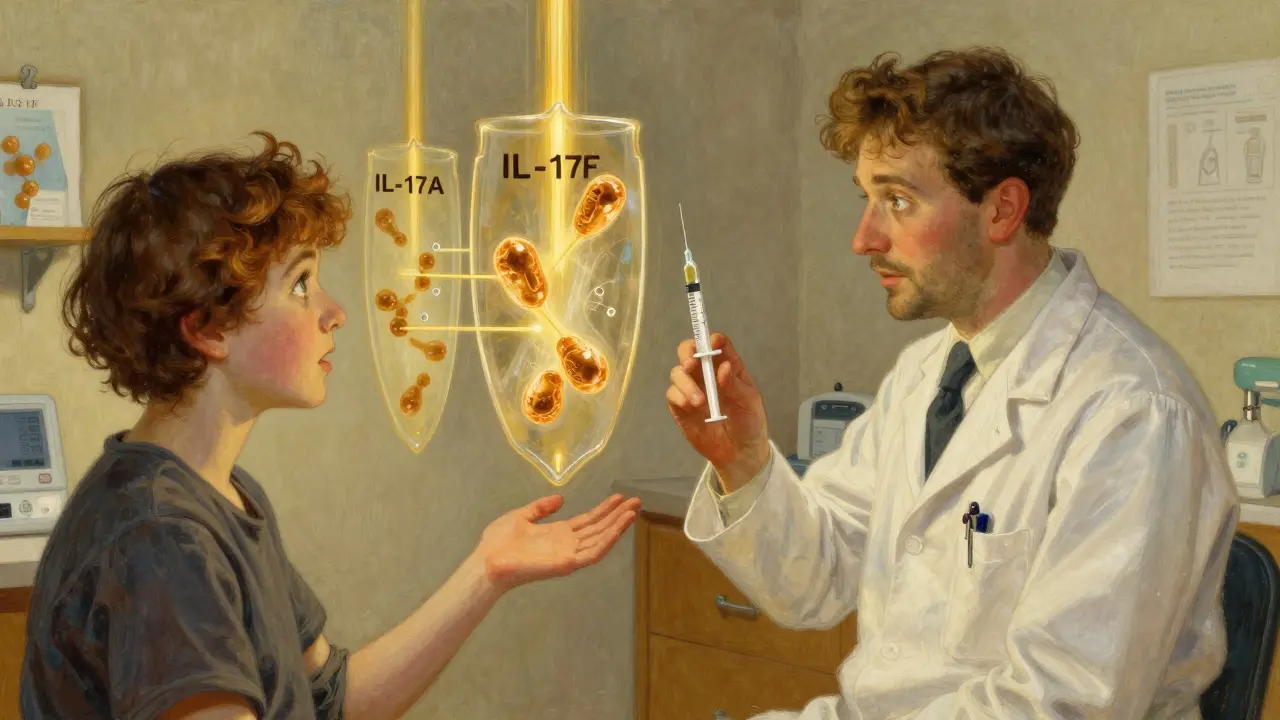
Then came biologics. These aren’t traditional drugs. They’re lab-made proteins that act like precision missiles, targeting specific parts of your immune system that go haywire in HS. Instead of blasting your whole body with steroids or antibiotics, biologics zero in on the troublemakers: cytokines like TNF-alpha, IL-17A, and IL-17F. These molecules drive the inflammation that makes HS so painful and persistent.
The first FDA-approved biologic for HS was adalimumab (Humira) in 2015. It blocks TNF-alpha, a key inflammatory signal. Since then, two more have joined the fight: secukinumab (Cosentyx) and bimekizumab (BIMZELX). Each targets IL-17, but differently. Secukinumab blocks only IL-17A. Bimekizumab blocks both IL-17A and IL-17F-two signals that work together to fuel HS flare-ups.
How Effective Are They? Real Numbers
It’s one thing to say a drug works. It’s another to see the numbers.
For adalimumab, clinical trials showed 41.8% of patients had at least a 50% reduction in abscesses and draining nodules after 12 weeks. That’s more than 4 in 10 people getting real relief. In the SUNSHINE trial, secukinumab hit 44.5% at week 16. But bimekizumab? In the BE HEARD I trial, it hit 66.9%-nearly 2 out of 3 patients saw major improvement.
And it’s not just about skin. People on biologics report fewer infections, less pain, and even better sleep. One patient on Reddit wrote: “After four weeks on secukinumab, I wore shorts for the first time in 12 years.”
Long-term results are even stronger. At one year, 56.4% of patients on secukinumab still had 50% or better improvement. For adalimumab, it was 48.7%. That’s a meaningful gap. And in patients with severe scarring, adalimumab still holds an edge-likely because TNF-alpha plays a bigger role in tissue damage over time.
Cost, Access, and Real-Life Challenges
These drugs aren’t cheap. In the U.S., adalimumab costs about $5,800 a month. Secukinumab is $6,200. Bimekizumab hits $6,900. Insurance often covers them-but not always. A 2024 report found only 45% of Medicaid patients got approval, compared to 82% of those with private insurance.
Out-of-pocket costs can still hit $1,200 a month for some. That’s why 33% of patients on Reddit and MyHSteam stopped treatment-not because it didn’t work, but because they couldn’t afford it.
Side effects are real, too. Injection site reactions happen in about 40% of users. Upper respiratory infections are common. A small number develop serious infections like tuberculosis or hepatitis B reactivation. That’s why doctors require blood tests for TB and hepatitis before starting any biologic. Heart failure is another risk-so if you have a weak heart, these drugs aren’t for you.
Who Should Get Biologics? The Rules
Not everyone with HS qualifies. Doctors use the Hurley staging system:
- Stage I: Single or a few isolated abscesses, no scarring or tunnels. Usually treated with antibiotics or lifestyle changes.
- Stage II: Recurrent abscesses with tunnels forming between them. This is where biologics usually start.
- Stage III: Widespread, interconnected tunnels and abscesses across large areas. Biologics help, but surgery often still needed.
Biologics are recommended for Stage II and III when other treatments fail. Experts like Dr. Amy Paller at Northwestern say: “Start early. Don’t wait until the scarring is permanent.”
Before starting, you’ll need:
- Tuberculosis screening
- Hepatitis B and C blood tests
- Heart function check
- Discussion about vaccines (you can’t get live vaccines while on biologics)
Response is checked at 12 weeks using the IHS4 score. If you’re not improving, your doctor may switch you to another biologic. About 1 in 3 people need to try more than one before finding the right fit.

What About the Future?
The pipeline is full. Three new biologics are in late-stage trials:
- Guselkumab (targets IL-23) showed 58.3% HiSCR50 in early results.
- Spesolimab (targets IL-36) hit 52.7% improvement.
- TAK-279 (a TYK2 inhibitor) had 55.1% response in Phase II.
And researchers are getting smarter. A 2024 study in Nature Communications identified a 12-gene signature that predicts who will respond to adalimumab-with 85% accuracy. That means someday, a simple blood test could tell you which drug will work best for you.
Combination therapy is also gaining ground. One 2024 study found that when bimekizumab was paired with surgical removal of damaged tissue, 89.2% of patients hit 50% improvement-far higher than either treatment alone.
What You Can Do Now
If you have HS and haven’t tried biologics yet, talk to a dermatologist who specializes in it. Not all do. Ask if your clinic has an HS program-78% of U.S. academic centers now do, up from just 32% in 2019.
Also, don’t underestimate lifestyle. Smoking doubles your risk of severe HS. Obesity makes it worse. Losing even 10% of body weight can reduce flares. Quitting smoking alone can cut your flare frequency by nearly half.
And if cost is a barrier, ask about patient assistance programs. AbbVie (Humira), Novartis (Cosentyx), and UCB (BIMZELX) all offer support for those who qualify.
HS isn’t just a skin condition. It’s a systemic disease with deep physical and emotional tolls. But for the first time, we have tools that don’t just mask symptoms-they change the course of the disease. The goal isn’t just to reduce nodules. It’s to help you live without fear, pain, or shame.
Can biologic therapy cure hidradenitis suppurativa?
No, biologics don’t cure HS. But they can put it into long-term remission for many people. Most patients need to stay on treatment indefinitely to keep symptoms under control. Stopping the drug often leads to flare-ups within months. Think of it like insulin for diabetes-it doesn’t fix the root cause, but it keeps the disease from destroying your life.
How long does it take for biologics to work for HS?
Most people start seeing improvement in 4 to 8 weeks. By week 12, doctors can tell if the drug is working. Some patients report dramatic relief in just 2 weeks, especially with IL-17 inhibitors like secukinumab. But full benefit often takes 3 to 6 months. Patience is key-this isn’t a quick fix.
Are biologics safe for long-term use?
Yes, for most people. Adalimumab has been used safely for over 20 years in psoriasis and rheumatoid arthritis. Long-term HS data (up to 5 years) shows sustained effectiveness with manageable risks. The biggest concerns are infections, especially if you’re also on steroids or have other health issues. Regular monitoring-blood tests, check-ins with your doctor-is essential. Most side effects are mild and don’t require stopping treatment.
Why is bimekizumab considered more effective than other biologics?
Bimekizumab blocks both IL-17A and IL-17F, two closely related inflammatory signals that work together in HS. Earlier drugs like secukinumab only blocked IL-17A. By hitting both, bimekizumab shuts down more of the inflammation pathway. In clinical trials, it achieved a 66.9% response rate at 16 weeks-higher than any other approved biologic. It’s also dosed less frequently: just once every 4 weeks after the first dose.
Can I use biologics if I’ve had surgery for HS?
Yes. In fact, combining biologics with surgery often gives the best results. Surgery removes the worst damaged tissue, while biologics prevent new lesions from forming. Many dermatologists now recommend starting biologics after surgery to reduce the chance of recurrence. Studies show this combo cuts flare-ups by nearly half compared to surgery alone.
Do biologics help with HS-related pain and fatigue?
Absolutely. Pain isn’t just from the abscesses-it’s from chronic inflammation. Biologics reduce systemic inflammation, which lowers pain levels and improves energy. In one study, patients reported their average pain score dropping from 7.2 to 2.4 on a 10-point scale within 4 weeks. Fatigue improved too. Many patients say they can finally sleep through the night, go back to work, or play with their kids without fear of a flare.


Kiruthiga Udayakumar
January 7, 2026 AT 14:25Let me just say this: if you're still using antibiotics for HS like it's 2010, you're not just behind the curve-you're burying your head in the sand. Biologics aren't optional anymore, they're essential. I've seen friends go from wheelchairs to hiking trails because someone finally listened to them. Stop blaming hygiene. Stop waiting for 'natural remedies.' This is medicine. And if your doctor won't prescribe it, find a new one. Period. 🚫🧴
tali murah
January 9, 2026 AT 00:05Oh, wonderful. Another $7,000/month miracle drug that only works if you’re rich, white, and have private insurance. Let’s not forget that 55% of Medicaid patients get denied. So what’s the real message here? ‘Sorry, your pain is too poor to treat.’ The pharmaceutical industry didn’t invent biologics to help people-they invented them to make shareholders cry tears of joy. And now we’re supposed to clap? 🙃
Elisha Muwanga
January 10, 2026 AT 22:13Look, I get that HS is terrible-but let’s not pretend this is some revolutionary breakthrough. We’ve had biologics for psoriasis for two decades. Now we’re just applying the same tech to another group of people who were ignored. Meanwhile, our VA hospitals still can’t get patients basic wound care. Why are we spending billions on drugs for the privileged few while veterans suffer in silence? Priorities, people. Priorities.
Maggie Noe
January 11, 2026 AT 11:37It’s wild how much our bodies are just… misunderstood ecosystems. HS isn’t a ‘skin problem’-it’s your immune system screaming because it’s been betrayed by genetics, stress, and a world that tells you to ‘just lose weight.’ Biologics don’t cure it, but they let you breathe again. I cried when I wore shorts for the first time in 15 years. Not because I was ‘fixed’-but because I was finally seen. 💔➡️💙
Jeffrey Hu
January 12, 2026 AT 05:17Actually, you’re all missing the point. The real issue isn’t the drugs-it’s the IHS4 scoring system. It’s outdated. It measures abscess count but ignores pain intensity, sleep disruption, and mental health impact. A patient could have 50% fewer nodules but still be suicidal. The FDA needs to update the metrics. Also, bimekizumab’s 66.9% response rate? That’s from a trial with strict inclusion criteria. Real-world effectiveness is probably closer to 40-45%. Don’t get sold on hype.
Jerian Lewis
January 13, 2026 AT 08:10I’ve been on adalimumab for 18 months. It works. But I’m tired. Tired of injections. Tired of explaining why I can’t go to the pool. Tired of people saying ‘it’s just acne.’ I don’t need a lecture on biologics. I just need someone to say ‘I get it.’ And if you’re lucky enough to have access? Use it. But don’t act like everyone else is lazy for not having it.
Patty Walters
January 14, 2026 AT 11:23Just wanted to add-smoking quit changed my life. I was on Humira and still flaring. Then I stopped. No joke, within 3 weeks, my flares cut in half. Also, if you’re struggling with cost, ask for the patient assistance program. AbbVie’s program helped me pay $5/month. Seriously. Just call them. They’re not perfect, but they care. 🙏
Phil Kemling
January 14, 2026 AT 15:31What if the real cure isn’t a drug at all, but a society that stops blaming people for their biology? HS isn’t a failure of willpower-it’s a failure of medical imagination. We treat the skin like it’s separate from the soul. But pain doesn’t live in a dermatome-it lives in the quiet spaces between breaths, in the silence after you say ‘I’m fine.’ Maybe the biologics are just giving us time to fix that.
Drew Pearlman
January 16, 2026 AT 00:36Hey, I just want to say-you’re not alone. I know it feels like the world doesn’t see you, like every mirror shows you something broken. But I’ve been there. I used to hide under long sleeves in 95-degree weather. I cried in the shower because the pain was too much to bear out loud. And then I found biologics. And now? I dance with my daughter in the kitchen at 2 a.m. because I finally feel like me again. It’s not perfect. It’s not easy. But it’s worth it. Keep going. You’re stronger than you think. And you deserve to feel good in your own skin. 💪❤️