ACE Inhibitor and ARB Risk Calculator
Medication Risk Assessment Tool
This tool estimates your risk of hyperkalemia, kidney injury, and other adverse effects when taking ACE inhibitors or ARBs. Based on clinical data from studies like ONTARGET and VA NEPHRON-D.
When it comes to managing high blood pressure, heart failure, or kidney damage from diabetes, doctors often turn to two types of medications: ACE inhibitors and ARBs. They’re both designed to calm down the renin-angiotensin system - a key player in how your body controls blood pressure and fluid balance. But here’s the catch: even though they target the same system, they don’t work the same way. And mixing them? That’s where things get risky.
How ACE Inhibitors and ARBs Work Differently
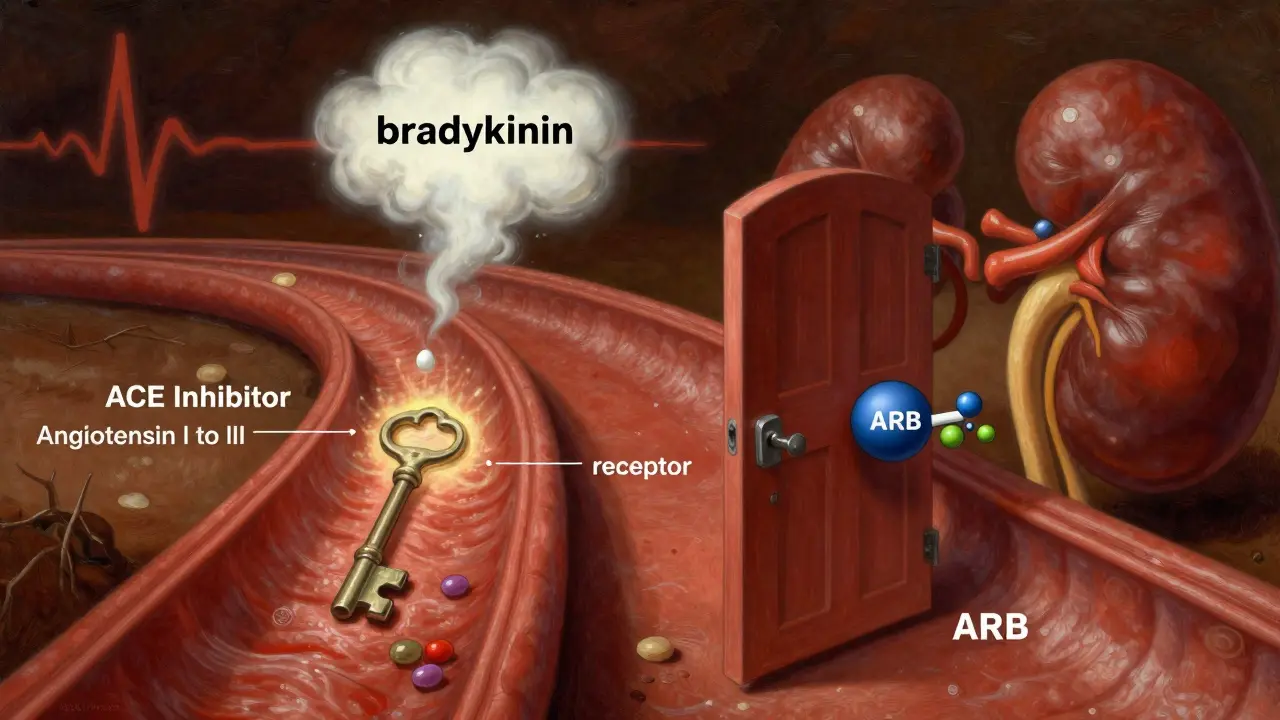
ACE inhibitors like lisinopril, enalapril, and ramipril block the enzyme that turns angiotensin I into angiotensin II. Angiotensin II is a powerful vasoconstrictor - it tightens blood vessels and raises blood pressure. By stopping its production, ACE inhibitors help relax vessels and reduce fluid retention.
ARBs - such as losartan, valsartan, and irbesartan - take a different route. Instead of stopping angiotensin II from being made, they block its receptors. Think of it like locking the door so angiotensin II can’t get in, even if it’s still floating around in the bloodstream.
This small difference matters. ACE inhibitors cause bradykinin to build up, which is why about 10-15% of people on them develop a dry, nagging cough. ARBs don’t do that. Their cough rate is only 3-5%. That’s why many patients switch from an ACE inhibitor to an ARB when the cough becomes unbearable.
The Big Risk: Combining Them
You might think, "If one works, two should work better." But that’s not true here. Multiple large studies have shown that combining an ACE inhibitor with an ARB doesn’t give you extra protection - it just adds danger.
The ONTARGET trial, published in the New England Journal of Medicine in 2008, followed over 25,000 high-risk patients. Those on both drugs had a 2.3% chance of needing dialysis due to kidney failure - more than double the 1.0% risk in those taking only ramipril. Hyperkalemia (dangerously high potassium) jumped from 2.5% to 5.5%. No improvement in heart attacks, strokes, or death.
A 2022 review in Hypertension looked at 12 clinical trials and confirmed the pattern. Combination therapy lowered blood pressure by only 3-5 mmHg more than one drug alone - but doubled the risk of hyperkalemia and raised kidney injury risk by 80%. The FDA and major medical groups now say this combo should be avoided outside of research settings.
When Might Doctors Still Consider It?
There’s one small group where some experts still consider combining them: non-diabetic patients with heavy proteinuria - over 1 gram per day - that won’t budge even on maximum ACE inhibitor doses. A 2022 report in Kidney International noted that in these rare cases, adding an ARB might cut protein loss by 40-60%.
But even then, it’s not a green light. Every patient on this combo needs weekly blood tests for potassium and kidney function. One nephrologist at Massachusetts General Hospital reported stopping the combo in 87% of her patients with diabetic kidney disease because of rising potassium or falling kidney function.
Most doctors won’t even try it. A 2023 survey of 317 primary care physicians found only 11% still used the combo - and only in patients with monthly lab monitoring. The rest stopped after the 2018 VA NEPHRON-D trial showed a 27% increase in serious side effects with no benefit.

Side Effects You Can’t Ignore
Both drugs can raise potassium levels by 0.3-0.5 mmol/L on average. For someone with kidney disease or diabetes, that’s enough to trigger dangerous heart rhythms. That’s why labs are checked within 1-2 weeks of starting or changing the dose, then every 3 months.
Acute kidney injury happens in 5-10% of high-risk patients - especially if they’re dehydrated, on diuretics, or have narrow kidney arteries. This isn’t rare. A Reddit thread from March 2024 had 78% of medical residents saying they’d seen someone hospitalized for hyperkalemia after being put on both drugs.
Angioedema - swelling of the face, lips, or throat - is rare but life-threatening. It happens in 0.1-0.7% of ACE inhibitor users, and about half that with ARBs. If you’ve had angioedema on one, you’re at higher risk on the other. That’s why switching from an ACE inhibitor to an ARB isn’t always safe - you might be reacting to the same underlying mechanism.
What to Do Instead
If your blood pressure isn’t controlled on one drug, don’t add the other. Instead, doctors are trained to add a mineralocorticoid receptor antagonist like spironolactone (12.5 mg daily). It cuts proteinuria by 30-40% with fewer risks than an ARB-ACE combo.
For heart failure patients with persistent high potassium, guidelines now suggest switching from an ACE inhibitor to an ARB - not adding one. A 2022 subanalysis showed a 17% drop in hospitalizations for high potassium when making the switch.
And for those who need stronger protection than either drug can offer alone, newer options like ARNIs (angiotensin receptor-neprilysin inhibitors) - such as sacubitril/valsartan - are now first-line for heart failure. They’ve proven better than ACE inhibitors alone in reducing death and hospitalization.
Switching Between Them: Don’t Rush
If you need to switch from an ACE inhibitor to an ARB (or vice versa), don’t just stop one and start the other the next day. The Cleveland Clinic recommends a 4-week washout period to avoid overlapping effects. But only 42% of prescribers actually follow this. That’s why some patients end up with sudden drops in blood pressure or spikes in potassium.
Also, don’t forget: ARBs had recalls between 2018 and 2020 due to cancer-causing nitrosamine impurities. Most of those issues are fixed now, but if you’re on valsartan, losartan, or irbesartan, make sure your pharmacy is using a clean batch.
Market Trends and What’s Next
In 2023, ACE inhibitors made up 58% of new RAS blocker prescriptions in the U.S., led by lisinopril (22.1 million prescriptions). ARBs like losartan came in second at 42%. But the trend is clear - fewer people are getting both drugs together. By 2028, experts predict less than 1% of prescriptions will involve this combo.
The future lies in smarter alternatives. The ongoing FINE-REWIND trial (NCT05192641) is testing whether half-dose combinations might be safe for kidney protection in diabetics. Results aren’t due until late 2026. Until then, the message is loud and clear: don’t mix them.
Can I take an ACE inhibitor and ARB together if my blood pressure is still high?
No. Combining these drugs increases your risk of kidney injury, dangerously high potassium, and even dialysis - without giving you better protection from heart attacks or strokes. If your blood pressure isn’t controlled, talk to your doctor about adding a diuretic, calcium channel blocker, or spironolactone instead.
Why do ACE inhibitors cause a cough but ARBs don’t?
ACE inhibitors block the enzyme that breaks down bradykinin, a substance that can irritate the airways. This buildup causes a dry, persistent cough in 10-15% of users. ARBs don’t affect bradykinin, so they rarely cause this side effect. If you get a cough on an ACE inhibitor, switching to an ARB often fixes it.
Is it safe to switch from an ACE inhibitor to an ARB?
Yes - and it’s often recommended if you have a cough or angioedema. But don’t switch overnight. A 4-week gap is ideal to avoid overlapping effects. Always check your kidney function and potassium levels 1-2 weeks after the switch.
Do ARBs work as well as ACE inhibitors for heart failure?
For heart failure with reduced ejection fraction, ACE inhibitors have stronger evidence for reducing death. Studies show a 23% risk reduction with ACE inhibitors versus 15% with ARBs. But ARBs are still effective - especially if you can’t tolerate ACE inhibitors. For most patients, either one is better than nothing.
What should I monitor if I’m on one of these drugs?
Check your serum potassium and creatinine (kidney function) 1-2 weeks after starting or changing the dose. Then every 3 months if you’re stable. If you have diabetes, kidney disease, or are on diuretics, you may need testing every month. Watch for dizziness, swelling, or irregular heartbeat - these could signal high potassium or low blood pressure.

Robert Petersen
February 12, 2026 AT 00:09Carla McKinney
February 12, 2026 AT 23:44Ojus Save
February 13, 2026 AT 01:29Luke Trouten
February 13, 2026 AT 14:59Gabriella Adams
February 15, 2026 AT 04:45Jonathan Noe
February 15, 2026 AT 11:29Jim Johnson
February 17, 2026 AT 04:58Vamsi Krishna
February 17, 2026 AT 20:59Brad Ralph
February 18, 2026 AT 01:55christian jon
February 18, 2026 AT 05:25Steve DESTIVELLE
February 18, 2026 AT 05:50Stephon Devereux
February 18, 2026 AT 21:37steve sunio
February 20, 2026 AT 01:44